The development of medical devices is expensive and time-consuming, especially when in-vivo testing is needed to characterize and validate the device. In medical device development, bench models are preferred for testing as they minimize variables and are cheaper and faster than in-vivo studies. Developers of patient temperature management systems incorporating warming and cooling garments (such as Belmont Medical Technologies Allon® and CritiCool®) did not previously have a bench model that could simulate in-vivo studies due to the complex nature of human thermal physiology. These medical devices typically control the patient’s core temperature during operating procedures or in intensive care. Developing bench models to simulate human thermal physiology will vastly improve patient temperature management by avoiding expensive testing and giving a repeatable model for engineers to develop better technologies.
A smaller field of patient temperature management called therapeutic hypothermia (TH) has a growing interest, especially in neonates with moderate to severe hypoxic-ischemic encephalopathy (HIE). Temperature management products induce TH in neonates by reducing the core temperature as low as 33⁰C (91.4⁰F), which has been shown to benefit cardiac and patients with brain injury. For neonatal patients, TH improves survival and neurodevelopment in neonates. During temperature management, it is essential to maintain temperature to deliver the correct therapy while cooling to therapeutic temperatures and rewarming to normothermic temperatures. Before this study, a physiological thermal model did not exist for neonates/infants, though one would significantly improve the development of temperature control systems.
An adaptive thermal manikin system was developed to simulate infants undergoing therapeutic hypothermia to address this need. An adaptive manikin system, i.e., a thermal manikin controlled by a human thermal model, is a test device that can simulate thermoregulation to accurately predict the core temperature and produce spatially varying skin temperatures and sweat rates consistent with that of the human body. An adaptive manikin can be used as a human surrogate in scenarios where human subject testing is either too time-consuming and/or not feasible due to the extreme nature of a given exposure.
Figure 1. Baby Thermal Manikin, Thermetrics LLC, (left) and its CAD representation (right)
The adaptive sweating thermal manikin system developed under this project was created by combining a customizable human thermal model (TAITherm Human Thermal Extension) with a commercially available sweating thermal manikin design representing the body build of a 9-month-old infant (Baby Thermal Manikin, Thermetrics LLC). The Baby Thermal Manikin was constructed using a thermally conductive carbon-epoxy shell with internal resistance wire heaters and precision skin temperature sensors. The manikin incorporates a sweating skin system, which utilizes a matrix of pores over the active surface of the manikin coupled with a computerized fluid delivery and a wicking fabric skin layer to distribute water over the surface of the manikin evenly.
The anthropometric description of the HTM (Human Thermal Model) was defined to be consistent with the segment lengths and surface areas derived from a CAD representation of the Baby Thermal Manikin. All additional information needed for fully describing the anthropometry of the infant HTM was derived from published literature and a high-resolution voxel model representation of an infant. The TAITherm infant computer model was tested by reproducing experiments described in the literature via pure virtual simulation of ambient conditions. This validated the model’s ability to predict the transient core temperature and mean skin temperature during a step change in ambient conditions and local skin temperatures and evaporation rates at various ambient conditions in relative steady-state.

Figure 2. Simulation of an infant undergoing therapeutic hypothermia using a human surrogate
Finally, the adaptive manikin system (ManikinPC) was tested within its primary use case by simulating an infant undergoing therapeutic hypothermia. Consistent with past studies on cooling neonate patients, the therapeutic device reduced the predicted core temperature in the adaptive manikin system to 33.5⁰C within 94 minutes. To quantify the full range of core temperature reduction that could be achieved in patients with varying levels of impeded thermoregulatory function due to anesthesia, testing, and simulation were performed by running the pure virtual model (TAITherm) and the manikin (Manikin) with and without vasoconstriction and shivering.
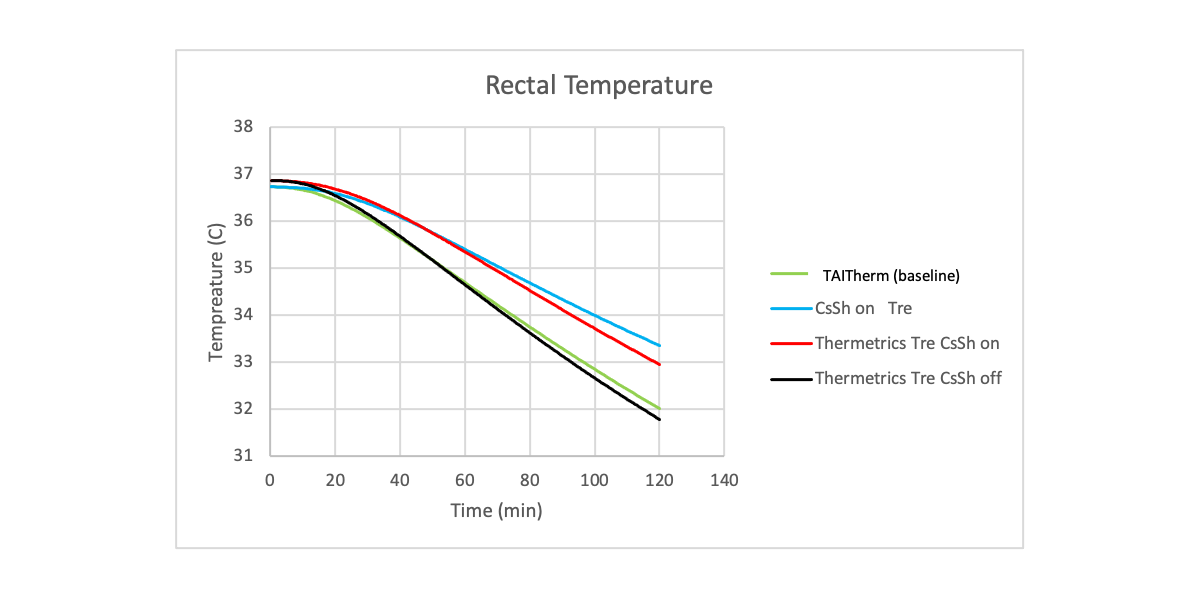
Figure 3. Predicted (TAITherm) vs. “measured” (ManikinPC) rectal temperature of an infant undergoing therapeutic hypothermia
This project demonstrates the value of a human surrogate test device for bench testing, developing patient temperature control systems, and improving the quality of temperature products. While this work is specific to temperature management products, the outcome of this study may also apply to other fields, such as thermal comfort models for testing clothing, diapers, bedding, and carriers, including car seats and strollers.
If you want to learn more about using TAITherm to maximize your human thermal comfort, please feel free to request a live demo of our software.
For more information about Thermetrics:

Blogs:
Get Comfy Quickly with ThermoAnalytics Human Thermal Comfort Software
https://blog.thermoanalytics.com/blog/get-comfy-quickly-thermoanalytics-human-thermal-comfort-software
Visit our website at suppport.thermoanalytics.com for
- FAQs
- Webinars
- Tutorials
Get help from our technical support team:

